source
Scientists from North Carolina State University have discovered a new phase of solid carbon, called Q-carbon. It is harder and brighter than another well-known solid phase of carbon – diamonds.
The intricate process
Researchers at North Carolina State University can create this new substance with a very intricate process. First, they start with a substrate, like sapphire, glass or a plastic polymer. This is covered with loose carbon. Then, they hit the loose carbon with a single laser pulse that lasts 200 nanoseconds – which is incredibly fast at 200 billionths of a second. Though it is short, that is enough time to heat carbon to about 3,700 degrees Celsius. That is close to double the heat that scientists believe made the first natural diamonds a billion years ago. The scientists then rapidly cool the carbon and forced the atoms into a special crystalline structure. This operation takes place at one atmosphere — the same pressure as the surrounding air.

{adinserter CNP5}
An amazing new find
“We’ve now created a third solid phase of carbon,” says Jay Narayan, the John C. Fan Distinguished Chair Professor of Materials Science and Engineering at NC State and co-author of the report published in the Journal of Applied Physics. “The only place it may be found in the natural world would be possibly in the core of some planets.” The result is so bright that it glows even in the dimmest light.
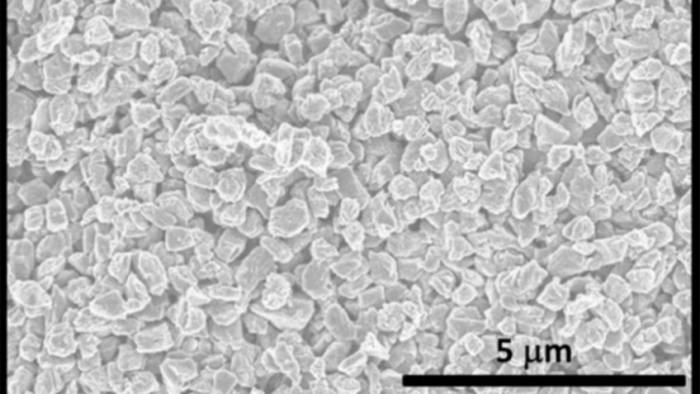
The possibilities are endless
This new material could become very useful. In addition to being harder than diamonds, Q-carbon is also ferromagnetic. Meaning, it can be magnetized. “We didn’t even think that was possible,” Narayan said. And since it is so bright, it could make electronic displays brighter and clearer.
“Q-carbon’s strength and low work-function — its willingness to release electrons — make it very promising for developing new electronic display technologies.”

It can help the medical industry
Perhaps the most exciting potential use of Q-carbon is that scientists “can create diamond nanoneedles or microneedles, nanodots, or large-area diamond films, with applications for drug delivery, industrial processes and for creating high-temperature switches and power electronics,” Narayan said.
“These diamond objects have a single-crystalline structure, making them stronger than polycrystalline materials. And it is all done at room temperature and at ambient atmosphere — we’re basically using a laser like the ones used for laser eye surgery. So, not only does this allow us to develop new applications, but the process itself is relatively inexpensive.”

There is still much to learn
According to Narayan, the reason they are creating diamond needles instead of making them out of Q-carbon is the following: “We are still in the early stages of understanding how to manipulate it…We don’t yet know how to make Q-carbon nanodots or microneedles. That’s something we’re working on.”